Method of electrolyser calculation in energyPRO
Input for the model
- Nominal efficiency of the Electrolyser [%] (relating hydrogen production to electricity consumption)
- Electric Capacity of the Electrolyser [MW]
Further optional inputs:
- Heat output [MW]
- Minimal load [MW]
General constants
- Molar weight of hydrogen: 1.00784 g/mol
- Molar weight of oxygen: 15.999 g/mol
- Lower heating value of hydrogen: 33.3 kWh/kg
Creating a load curve for an electrolyser
Since an electrolyser is very flexible to change its load, it is important to be able to know how the efficiency varies with the applied load. The efficiency in this document is related to, as suggested by Buttler et al. (2018) for energy applications, the lower heat value of hydrogen. The relative change in efficiency is however assumed to be similar. For the two mentioned technologies different load curves are assumed:
| [%] of nominal load | Alkaline Electrolysis (AE) [%] of nominal efficiency | Polymer Electrolyte Membrane Electrolysis (PEM) [%] of nominal efficiency |
|---|---|---|
| 25 (AE)/ 27 (PEM) | 105 | 115 |
| 100 | 100 | 100 |
The data point in the middle refers to the peak efficiency of the electrolyser system, also referred to as η_(\(H_2\),out,peak). It is seen below as the points where both curves show the highest efficiency, close after 20 % of their nominal load.
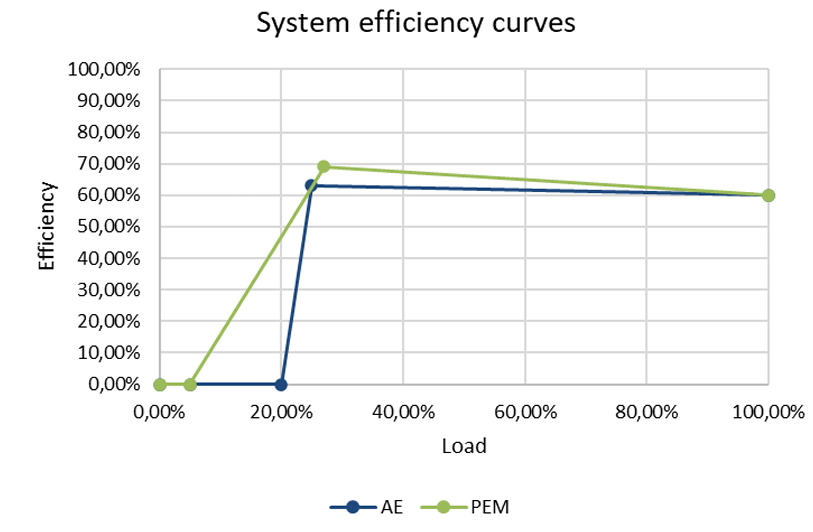
The shown efficiency curves relate both to AE and PEM with 60 % nominal efficiency, which is entered by the user. As seen the highest efficiency is reached in part load reaching 63% and 69% of efficiency respectively as a result of multiplying the nominal efficiency with the second row of the table above. This happens because an electrolysers cells are declining in efficiency with higher loads, while an electrolyser’s auxiliaries are increasing in efficiency with increasing load. The minimal loads are specified by the user to be 5 % of nominal load for the PEM and 20 % for the AE in the graph above.
Hydrogen and Oxygen Production and Water Consumption
The hydrogen production is modelled by multiplying on the electricity consumption with a factor of how much electricity is converted into hydrogen directly. This factor is referred to as efficiency.
The oxygen output is calculated based on the hydrogen output. Therefore, the mass balance to the chemical reaction of splitting water (\(2H_2O\)→2 \(H_2\)+ 2\(O_2\)) is used to calculate the amount of oxygen produced per kg of produced hydrogen. The oxygen output is calculated according to following equation:
The amount of water consumption is also determined based on the hydrogen production, by using the mass balance to the chemical reaction of splitting water.
Heat output
The heat production is modelled as absolute value. To prevent that the overall system efficiency will increase above 100%, the heat output at nominal load should not exceed:
Where:
\(η_{H_2,out,peak}\) is the highest possible efficiency of electric input to hydrogen production (LHV) and not the nominal efficiency.
\(E_{cap}\) is the nominal electric capacity of the electrolyser
This is because the model assumes the heat output to be constant from the point of maximum efficiency to nominal efficiency. Thereby, this heat output together with the hydrogen efficiency should not exceed the input of electricity.